Is Internal Energy The Same As Mechanical Energy . so no, internal energy and thermal energy is not at all the same. It is the ability to do work and can be either kinetic. internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. Learn how to calculate internal. internal energy is the sum of the kinetic and potential energies of the molecules in a system. the first law of thermodynamics states that the change in internal energy of a system equals the net. mechanical energy is the energy that is possessed by an object due to its motion or due to its position. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. See examples and graphs of. Internal energy is everything and includes.
from overallscience.com
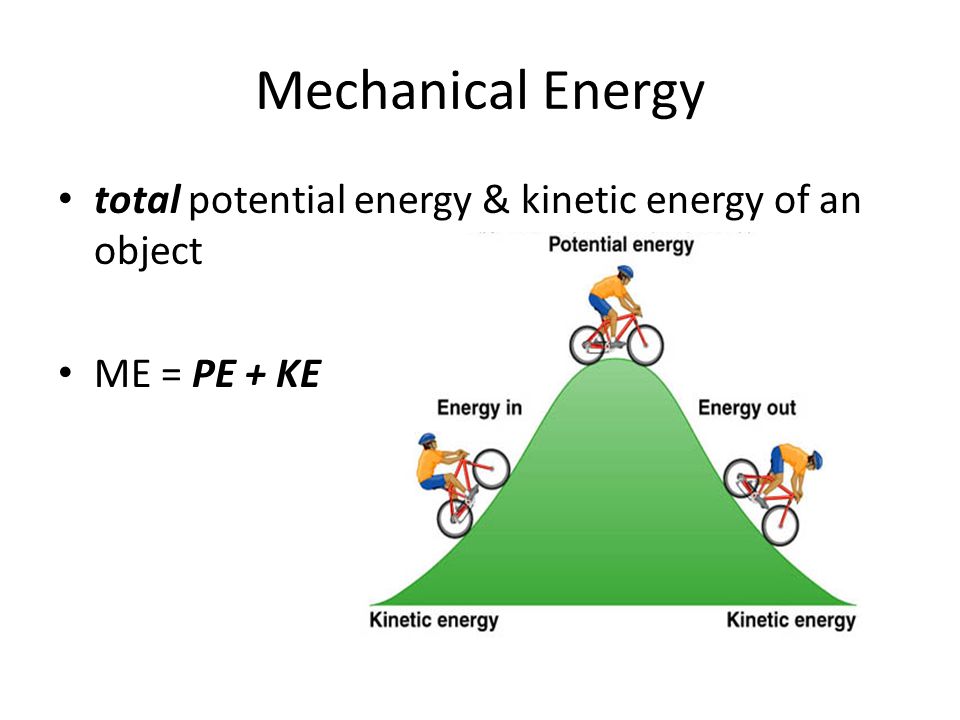
mechanical energy is the energy that is possessed by an object due to its motion or due to its position. the first law of thermodynamics states that the change in internal energy of a system equals the net. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. It is the ability to do work and can be either kinetic. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. internal energy is the sum of the kinetic and potential energies of the molecules in a system. so no, internal energy and thermal energy is not at all the same. See examples and graphs of. internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. Internal energy is everything and includes.
Mechanical energy and It’s types Overall Science
Is Internal Energy The Same As Mechanical Energy See examples and graphs of. so no, internal energy and thermal energy is not at all the same. the first law of thermodynamics states that the change in internal energy of a system equals the net. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. internal energy is the sum of the kinetic and potential energies of the molecules in a system. See examples and graphs of. It is the ability to do work and can be either kinetic. mechanical energy is the energy that is possessed by an object due to its motion or due to its position. Internal energy is everything and includes. internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. Learn how to calculate internal.
From www.thephysicspoint.com
What is Mechanical Energy? Definition, Examples, Formula and Types Is Internal Energy The Same As Mechanical Energy internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. Learn how to calculate internal. See examples and graphs of. internal energy is the sum of the kinetic and potential energies of the molecules in a system. internal energy is the total of the kinetic and potential energy. Is Internal Energy The Same As Mechanical Energy.
From www.tec-science.com
Internal energy & first law of thermodynamics tecscience Is Internal Energy The Same As Mechanical Energy mechanical energy is the energy that is possessed by an object due to its motion or due to its position. Internal energy is everything and includes. the first law of thermodynamics states that the change in internal energy of a system equals the net. Learn how to calculate internal. so no, internal energy and thermal energy is. Is Internal Energy The Same As Mechanical Energy.
From www.slideserve.com
PPT Work and Energy PowerPoint Presentation, free download ID3522834 Is Internal Energy The Same As Mechanical Energy Internal energy is everything and includes. It is the ability to do work and can be either kinetic. See examples and graphs of. Learn how to calculate internal. so no, internal energy and thermal energy is not at all the same. mechanical energy is the energy that is possessed by an object due to its motion or due. Is Internal Energy The Same As Mechanical Energy.
From engineerfix.com
What Is Mechanical Energy? Examples, Definition, And Some FAQs Is Internal Energy The Same As Mechanical Energy It is the ability to do work and can be either kinetic. mechanical energy is the energy that is possessed by an object due to its motion or due to its position. Learn how to calculate internal. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. so. Is Internal Energy The Same As Mechanical Energy.
From engineerfix.com
What Is Mechanical Energy? Examples, Definition, And Some FAQs Is Internal Energy The Same As Mechanical Energy See examples and graphs of. so no, internal energy and thermal energy is not at all the same. Internal energy is everything and includes. internal energy is the sum of the kinetic and potential energies of the molecules in a system. It is the ability to do work and can be either kinetic. Learn how to calculate internal.. Is Internal Energy The Same As Mechanical Energy.
From thepowerfacts.com
Law of Conservation of Mechanical Energy Examples (Formula & Definition Is Internal Energy The Same As Mechanical Energy It is the ability to do work and can be either kinetic. internal energy is the sum of the kinetic and potential energies of the molecules in a system. so no, internal energy and thermal energy is not at all the same. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic. Is Internal Energy The Same As Mechanical Energy.
From eduinput.com
What is Internal EnergyDefinition And Example Is Internal Energy The Same As Mechanical Energy mechanical energy is the energy that is possessed by an object due to its motion or due to its position. internal energy is the sum of the kinetic and potential energies of the molecules in a system. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. Learn. Is Internal Energy The Same As Mechanical Energy.
From www.examples.com
Mechanical Energy 20+ Examples, How to Calculate Is Internal Energy The Same As Mechanical Energy It is the ability to do work and can be either kinetic. Internal energy is everything and includes. so no, internal energy and thermal energy is not at all the same. See examples and graphs of. Learn how to calculate internal. internal energy is the sum of all the microscopic energies of a system, such as kinetic and. Is Internal Energy The Same As Mechanical Energy.
From www.sciencefacts.net
Mechanical Energy Definition, Types, Examples, and Formula Is Internal Energy The Same As Mechanical Energy learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. the first law of thermodynamics states that the change in internal energy of a system equals the net. See examples and graphs of. internal energy is the sum of all the microscopic energies of a system, such as. Is Internal Energy The Same As Mechanical Energy.
From thescienceteacher.co.uk
Internal energy teaching resources the science teacher Is Internal Energy The Same As Mechanical Energy See examples and graphs of. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. internal energy is the sum of the kinetic and potential energies of the molecules in a system. Internal energy is everything and includes. the first law of thermodynamics states that the change in. Is Internal Energy The Same As Mechanical Energy.
From eschool.iaspaper.net
Mechanical Energy Eschool Is Internal Energy The Same As Mechanical Energy mechanical energy is the energy that is possessed by an object due to its motion or due to its position. It is the ability to do work and can be either kinetic. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. internal energy is the total of. Is Internal Energy The Same As Mechanical Energy.
From overallscience.com
Mechanical energy and It’s types Overall Science Is Internal Energy The Same As Mechanical Energy mechanical energy is the energy that is possessed by an object due to its motion or due to its position. Learn how to calculate internal. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. internal energy is the sum of the kinetic and potential energies of the. Is Internal Energy The Same As Mechanical Energy.
From slidetodoc.com
WorkEnergy Theorem and Conservation of Mechanical Energy From Is Internal Energy The Same As Mechanical Energy mechanical energy is the energy that is possessed by an object due to its motion or due to its position. See examples and graphs of. Learn how to calculate internal. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. the first law of thermodynamics states that the. Is Internal Energy The Same As Mechanical Energy.
From quickelectricity.com
What Are the Main Types of Energy? Quick Electricity Is Internal Energy The Same As Mechanical Energy internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. Learn how to calculate internal. the first law of thermodynamics states that the change in internal energy of a. Is Internal Energy The Same As Mechanical Energy.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID5988619 Is Internal Energy The Same As Mechanical Energy See examples and graphs of. the first law of thermodynamics states that the change in internal energy of a system equals the net. mechanical energy is the energy that is possessed by an object due to its motion or due to its position. Internal energy is everything and includes. so no, internal energy and thermal energy is. Is Internal Energy The Same As Mechanical Energy.
From hallerenee.blogspot.com
Define Mechanical Energy And Its Types Hallerenee Is Internal Energy The Same As Mechanical Energy It is the ability to do work and can be either kinetic. the first law of thermodynamics states that the change in internal energy of a system equals the net. internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. learn how to calculate the work, heat transfer,. Is Internal Energy The Same As Mechanical Energy.
From www.researchgate.net
The diagram of the conservation of mechanical energy and total energy Is Internal Energy The Same As Mechanical Energy internal energy is the sum of all the microscopic energies of a system, such as kinetic and potential energy. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system.. Is Internal Energy The Same As Mechanical Energy.
From eduinput.com
What is Internal EnergyDefinition And Example Is Internal Energy The Same As Mechanical Energy Learn how to calculate internal. learn how to calculate the work, heat transfer, and internal energy change of a thermodynamic system in different processes. internal energy is the total of the kinetic and potential energy of the molecules and atoms in a system. See examples and graphs of. Internal energy is everything and includes. mechanical energy is. Is Internal Energy The Same As Mechanical Energy.